Tissue regeneration has become a central pillar of modern periodontology, implantology, and oral surgery. In recent decades, an impressive arsenal of biomaterials, devices, and biologically active substances has been introduced to the clinical workflow, offering clinicians numerous regenerative options. These include enamel matrix derivatives (EMDs) such as amelogenins, recombinant growth factors, bone morphogenetic proteins (BMPs), and autologous platelet concentrates. Despite their effectiveness, each of these tools presents specific limitations in terms of cost, technique sensitivity, and regulatory complexity.
Amelogenins, for example, while effective in certain periodontal regenerative contexts, suffer from biological instability when exposed to blood contamination—a frequent occurrence in surgery—which dramatically reduces their efficacy. BMPs, on the other hand, have demonstrated extraordinary regenerative potential but are associated with prohibitive costs and a risk profile that includes ectopic bone formation. Growth factors such as PDGF or FGF require extraction from blood or recombinant production under strict conditions, often encountering legal and logistical barriers in clinical use.
In this context, cross-linked hyaluronic acid (HA) is emerging as a promising alternative. It is a widely available, well-tolerated biomolecule with strong bioadhesive, hydrophilic, and anti-inflammatory properties, and now, as growing evidence shows, with real potential in promoting cellular responses that are essential to tissue regeneration. HA offers the potential for cost-effective, simplified, and minimally invasive protocols, aligning with current trends in oral surgery and periodontal therapy.
The in vitro study by Asparuhova et al. (2019) demonstrated that both native and cross-linked HA significantly promoted the proliferation and migration of human oral fibroblasts, alongside increased expression of genes linked to collagen synthesis (e.g., COL3A1) and anti-scarring pathways (e.g., TGF-β3). Notably, HA achieved these effects without inducing a pro-inflammatory phenotype, suggesting a safe and targeted activation of the healing cascade.
Building upon this, the recent investigation by Hakki, Bosic, and Sculean (2024) sheds light on HA’s regenerative influence on cementoblasts—key cells in periodontal regeneration. Their data showed that HA not only stimulates cementoblast proliferation and migration but also enhances mineralization, as confirmed by upregulation of ALP, BSP, Runx2, and other markers, and activation of the TGF-β/Smad and Wnt/β-catenin pathways. Particularly significant is the upregulation of CEMP-1 and CAP, cementum-specific proteins, indicating a direct role for HA in cementogenesis.
Clinical relevance is further reinforced by Božić et al. (2021), who reported significant improvements in CAL gain and PPD reduction when cross-linked HA was applied in combination with porcine bone graft in intrabony defects. These results support the hypothesis that HA not only serves as a scaffold but also modulates local wound healing biology by favoring fibroblast and osteogenic cell recruitment.
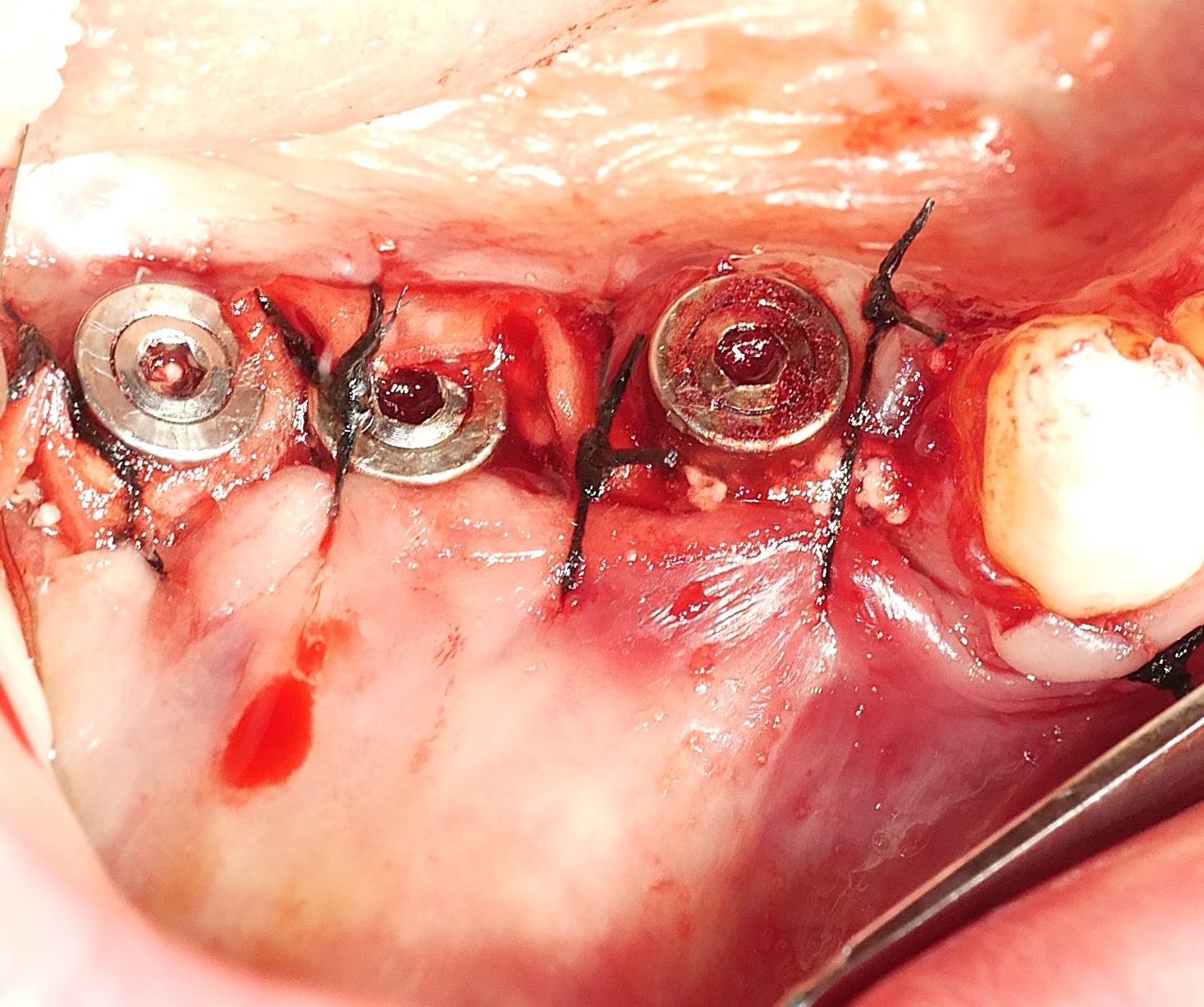
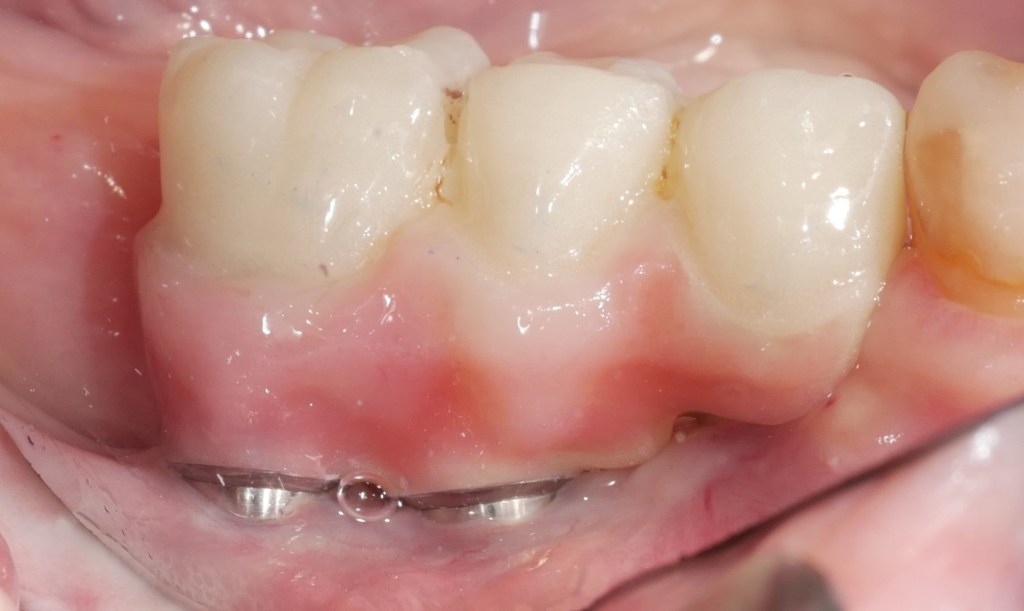
Hard and soft tissue augmentation by fibroblastic stimulation
Additional support comes from earlier findings by David-Raoudi et al. (2008), who explored how different HA molecular weights affect fibroblast behavior. They concluded that both native and fragmented HA stimulate collagen expression and influence the cellular phenotype through pathways involving CD44 and MAPK signaling. These results are consistent with those seen using cross-linked formulations, which prolong tissue retention and amplify biological signaling.
Taken together, this body of evidence positions cross-linked hyaluronic acid as a promising and versatile bioactive tool in oral regenerative procedures. Unlike other more complex or expensive biological agents, HA provides a straightforward and reproducible clinical approach. It supports soft and hard tissue healing, modulates inflammation, and now, as shown, actively promotes cementoblastic and fibroblastic functions essential for successful periodontal and peri-implant regeneration.
In light of this, future clinical trials are not only warranted but essential. Such investigations should aim to refine HA dosing strategies, define indications in surgical versus non-surgical contexts, and explore synergistic effects with other regenerative materials. The translation of these findings into everyday practice could offer clinicians a powerful, cost-effective solution for tissue regeneration in an era increasingly characterized by economic pressure and patient demand for minimally invasive care.
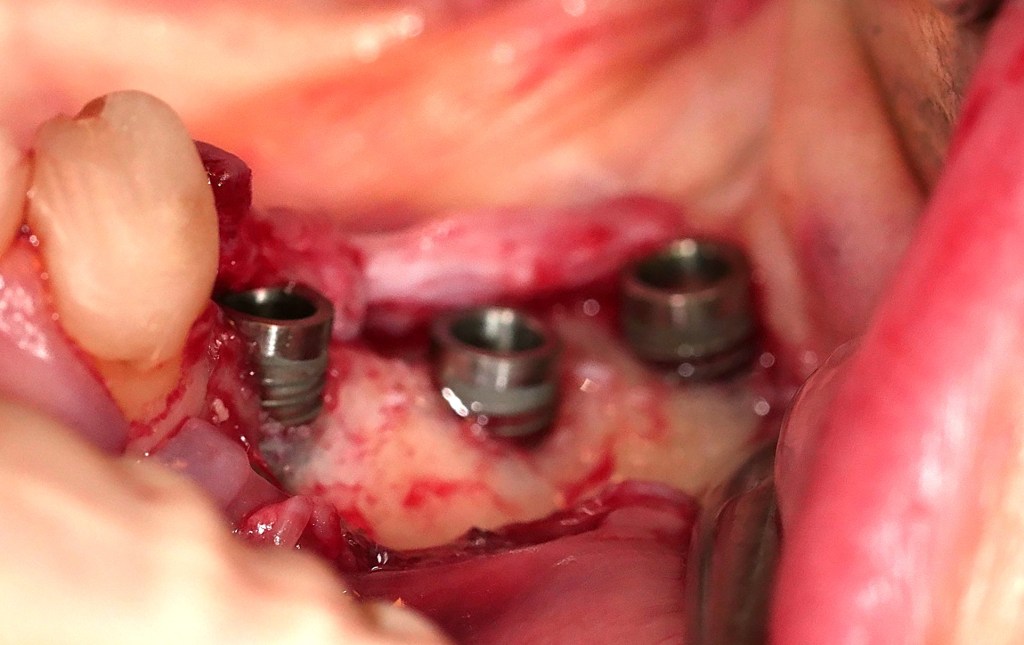
Cross-linked HA appears to meet the gold standard of the next-generation biomaterial: effective, accessible, easy to use, and biologically meaningful. It is time to expand its clinical application and validate, through rigorous clinical research, its place among the foundational tools of regenerative dentistry.1
References:
- Asparuhova MB, et al. J Periodont Res. 2019;54(1):33–45. doi:10.1111/jre.12602.
- Hakki SS, et al. J Periodont Res. 2024;59(1):63–73. doi:10.1111/jre.13201.
- Božić D, et al. Materials. 2021;14(22):6795. doi:10.3390/ma14226795.
- David-Raoudi M, et al. Wound Repair Regen. 2008;16(2):274–287. doi:10.1111/j.1524-475X.2007.00342.x.
- Text and images can be reproduced only by citing this article, the link and the author. Images and text are personal and cannot be reproduced separately without the author’s consent ↩︎
Leave a comment